Challenges Of Maintaining Cold Chain

Dr. Shyam P Lohani
The coronavirus pandemic has left no country untouched. The outbreak of this disease may still easily and almost rapidly overwhelm the already overstretched healthcare systems of the developing economies. Amid this pandemic, a large number of people is expecting to have effective therapeutics soon. We hope to have vaccines effective to prevent ourselves from the development of coronavirus disease, and at the same time, effective drugs to treat COVID-19 and its complications.
At this juncture of unfathomable sufferings of humanity from COVID-19, we are hopeful that the coronavirus-induced affliction will soon be over and we will return to our normal life. This hope is not anecdotal and based on a declining number of cases in some parts of the world, less number of reported deaths due to viral infection, a greater understanding of virology and its transmission, and news on the initial success of drugs and vaccines against the novel coronavirus.
Challenges
The power of vaccines has been proven historically as highly lethal influenza virus, polio, measles, and other viral outbreaks have been brought under control or effectively eliminated. However, we should not forget that vaccine in itself is not a panacea. The long and continuous battle begins with the development of an effective vaccine. An estimated 60-70 per cent of all people in the world should be vaccinated for effective immunisation drive. The endeavour of mass vaccination requires massive manufacturing, transportation logistics as well as strong cold chain systems.
The unparalleled speed with which many pharmaceutical companies are working to develop an effective vaccine is praiseworthy. The sooner the vaccine is developed the better it is for humanity to get relief from the sufferings. It is here important to have a similar kind of urgency to develop effective inventory management, vaccine distribution networks, and cold chain systems along with vaccine development to defeat the COVID-19 pandemic.
The COVID-19 vaccine, like most other vaccines, will need to be kept cold from the point it leaves the manufacturer to the time it is administered. To maintain the vaccine’s effectiveness, the cold chain should be precisely controlled within a recommended temperature range until it reaches the end-users. The experiences from many countries in the world have revealed that a significant proportion of the vaccines are wasted mainly in low- and middle-income countries owing to inadequate vaccine cold chain systems.
Depending on where vaccines are manufactured, it may take long-distance and time to travel until they are administered. As the vaccines are temperature sensitive, the entire journey from the manufacturer to the end-users require constant refrigeration. The exposure to both hot and freezing temperatures causes loss of their potency and must be discarded immediately to prevent the use of ineffective and unsafe antigen.
It has been estimated that billions of people worldwide require a coronavirus vaccine and may need up to two doses of it. The foreseeable massive mass vaccination efforts are destined to require substantial cold chain facilities worldwide. The current cold chain capability, even in developed countries, is not optimum and needs to scale up which is a daunting task.
Vaccines are biological products and thus, it is essential to maintain specific temperatures for a vaccine to retain its potency. Almost all vaccines are labelled to be stored and transported between 2˚ to 8˚Centigrade (36˚to 46˚Fahrenheit) at all times from manufacturing to administration to the people.
The majority of COVID-19 vaccines under development like the Moderna, Pfizer, and Genova Biopharma’s vaccines are new RNA-based vaccines. If they get too warm or too cold, they lose their potency. Moderna’s vaccine requires a storage temperature of minus 4 degrees Fahrenheit, Pfizer’s vaccine candidate requires a storage temperature of minus 94 degrees Fahrenheit, whereas Gennova Bophirima’s mRNA candidate requires storage at minus 70 degrees Centigrade. These temperatures are not easy to maintain accurately.
Preparedness
Once the vaccines are developed, the transporting them safely to the masses will be one of the biggest obstacles to cross as they require very specific temperature conditions, some as low as minus 70 degrees Centigrade. Planes, trucks, cold storage units will have to equip themselves to transport and maintain vaccines at such low temperatures, and glass vials, containers with the vaccines will also need to be packaged to withstand such icy conditions. A huge quantity of dry ice will also be necessary for maintaining temperatures. Storage and transportation of vaccines might just be the biggest challenge after their production and safety trials.
The lead developers are inexperienced in manufacturing large scale of vaccine; therefore, timely planning is required to ensure enough supply and distribution among large populations. Moreover, as billions of doses are required and to be distributed, strong coordination and cooperation among vaccine developers, policymakers, and regulators, private and public institutions, and governments will become vital. The equitable distribution of vaccine to the resource-limited yet affected areas warrants additional consideration. There has been concern already raised about the cold chain maintenance capability of resource-limited countries.
The world was not prepared for the arrival of COVID-19 and is experimenting and learning the way out of this crisis. Hoping to have vaccines in the foreseeable future, there is now no excuse for being inadequately prepared to address the upcoming challenges such as cold-chain maintenance. With the development of required supply chain management and training of staff, we can be ready to start immunising millions of people in our country and bring life back to old normal.
(A Professor, Lohani is the founder and academic director of Nobel College. lohanis@gmail.com)
Recent News

Do not make expressions casting dout on election: EC
14 Apr, 2022
CM Bhatta says may New Year 2079 BS inspire positive thinking
14 Apr, 2022
Three new cases, 44 recoveries in 24 hours
14 Apr, 2022
689 climbers of 84 teams so far acquire permits for climbing various peaks this spring season
14 Apr, 2022
How the rising cost of living crisis is impacting Nepal
14 Apr, 2022
US military confirms an interstellar meteor collided with Earth
14 Apr, 2022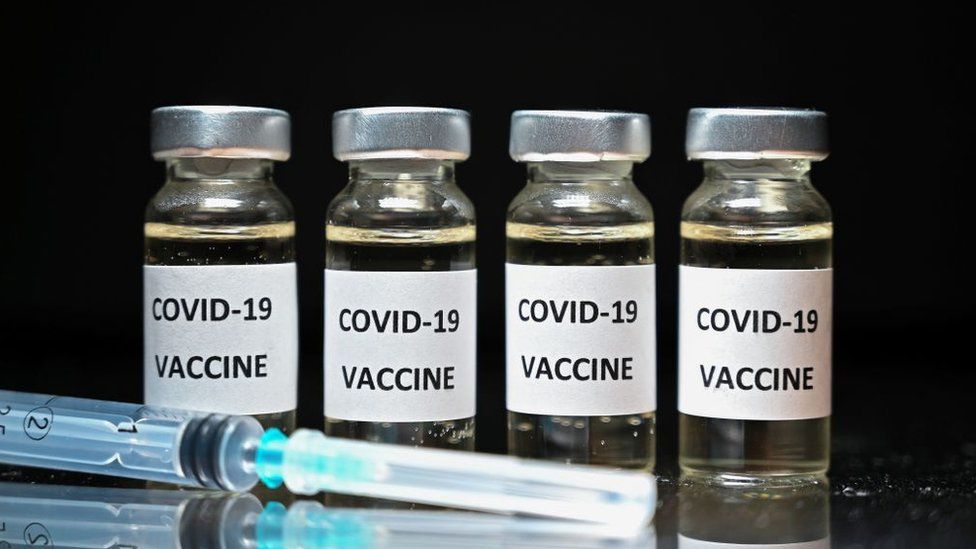
Valneva Covid vaccine approved for use in UK
14 Apr, 2022
Chair Prachanda highlights need of unity among Maoist, Communist forces
14 Apr, 2022
Ranbir Kapoor and Alia Bhatt: Bollywood toasts star couple on wedding
14 Apr, 2022
President Bhandari confers decorations (Photo Feature)
14 Apr, 2022