WHO recommends two new drugs for COVID-19 patients
By A Staff Reporter
Kathmandu, Jan. 15: The drug baricitinib, a type of drug known as a Janus kinase (JAK) inhibitor, also used to treat rheumatoid arthritis, is strongly recommended for the patients with severe or critical COVID-19 in combination with corticosteroids, said a WHO Guideline Development Group of international experts in The BMJ today.
Issuing a press statement on Friday, the WHO stated that their strong recommendation was based on moderate certainty evidence that it improves survival and reduces the need for ventilation, with no observed increase in adverse effects.
The WHO experts noted that baricitinib has similar effects to other arthritis drugs called interleukin-6 (IL-6) inhibitors so, when both are available, they suggested choosing one based on cost, availability, and clinician experience. It is not recommended to use both drugs at the same time.
However, the experts have advised against the use of two other JAK inhibitors (ruxolitinib and tofacitinib) for patients with severe or critical COVID-19 because low certainty evidence from small trials failed to show benefit and suggested a possible increase in serious side effects with tofacitinib.
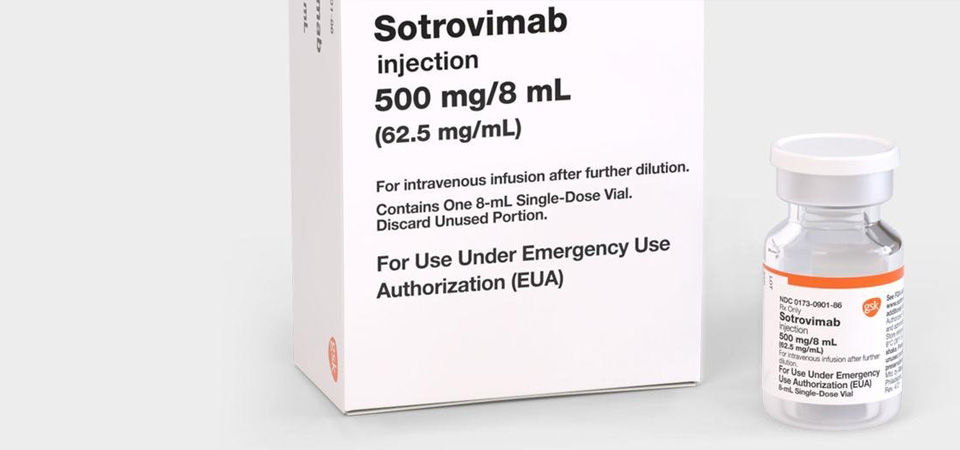
In the same guideline update, WHO has also made a conditional recommendation for the use of the monoclonal antibody sotrovimab in patients with non-severe COVID-19, but only in those at highest risk of hospitalisation, reflecting trivial benefits in those at lower risk.
A similar recommendation has been made by WHO for another monoclonal antibody drug (casirivimab-imdevimab). The experts also noted that there were insufficient data to recommend one monoclonal antibody treatment over another, and they acknowledged that their effectiveness against new variants like omicron was still uncertain.
As such, they said that the guidelines for monoclonal antibodies would be updated when additional data become available. According to the WHO, these recommendations are based on new evidence from seven trials involving over 4,000 patients with non-severe, severe, and critical COVID-19 infection.
They are part of a living guideline, developed by the World Health Organisation with the methodological support of MAGIC Evidence Ecosystem Foundation, to provide trustworthy guidance on the management of COVID-19 and help doctors make better decisions with their patients.
Recent News

Do not make expressions casting dout on election: EC
14 Apr, 2022
CM Bhatta says may New Year 2079 BS inspire positive thinking
14 Apr, 2022
Three new cases, 44 recoveries in 24 hours
14 Apr, 2022
689 climbers of 84 teams so far acquire permits for climbing various peaks this spring season
14 Apr, 2022
How the rising cost of living crisis is impacting Nepal
14 Apr, 2022
US military confirms an interstellar meteor collided with Earth
14 Apr, 2022
Valneva Covid vaccine approved for use in UK
14 Apr, 2022
Chair Prachanda highlights need of unity among Maoist, Communist forces
14 Apr, 2022
Ranbir Kapoor and Alia Bhatt: Bollywood toasts star couple on wedding
14 Apr, 2022
President Bhandari confers decorations (Photo Feature)
14 Apr, 2022