Remdesivir being used for research purpose with consent of patients
By Sampada A. Khatiwada
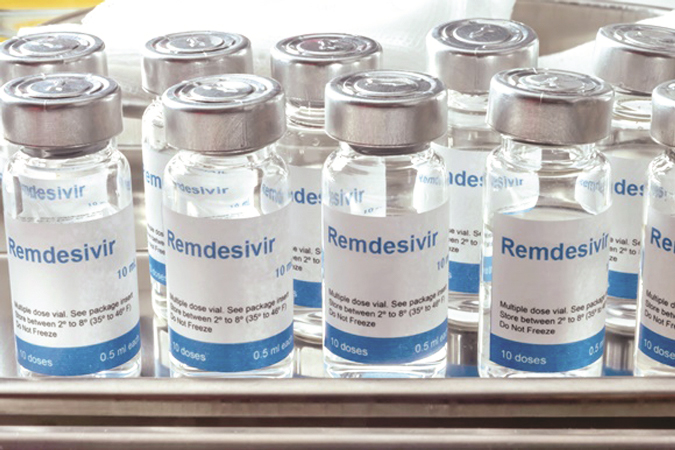
Kathmandu, Aug. 24: The availability of Remdesivir, a broad-spectrum anti-viral medicine, in Nepal, has raised the hopes of curing the COVID-19 infected patients, especially those who are in critical condition. The government, however, has limited the use and distribution of the Remdesivir as the medicine is still under trial.
Dr. Pradeep Gyawali, member secretary of the Nepal Health Research Council (NHRC) said, "The Remdesivir anti-viral is available in COVID-19 dedicated hospitals specified by the government."
The protocols and guidelines set forth by the NHRC, in direct surveillance of doctors, must be followed while medicating the virus patients with Remdesivir. "Since the medicine is under research and investigation, consent of patients is vital to put Remdesivir into use," added Dr. Gyawali.
He said that the medicine could be used only for the purpose of research and investigation, with approval of the Department of Drugs Administration (DDA). "Use and distribution of Remdesivir without approval of DDA would be punishable by law," added Dr. Gyawali.
According to Santosh KC, information officer and senior drug administrator at the DDA, the recently imported vials of Remdesivir were being used only in level-2 and level-3 COVID-19 dedicated hospitals.
“Currently, import of this anti-viral injection has been allowed only from three Indian companies, Cipla, Mylan and Hetero. An agreement was made on August 15 to allow import of 2000 vials of Remdesivir from each of these three companies,” said KC.He added that additional vials of Remdesivir would be brought on the basis of its effectiveness.
“Since this is not the ‘ultimate’ vaccine against COVID-19, the medicine is currently available only at COVID-19 special hospital. The medication is being administered only to those virus infected patients who are admitted to the Intensive Care Unit (ICU) with their consent,” added KC.
“The medicine is not available for asymptomatic virus patients. It is given only to those patients who are serious, only if they consent to being medicated with it, abiding by the standards set
by the NHRC,” said the senior drug administrator at the DDA. KC also clarified that the medicine would be available at the same costs the companies from which they are brought charge.
Meanwhile, the NHRC has already given permission for clinical trial of Remdesivir in Nepal. Being a COVID-19 dedicated hospital, the TU Teaching Hospital, among others, has been medicating the virus infected patients with Remdesivir only with their consent.
“We have been providing the injection to a couple of COVID-19 patients,” said Dr. Santa Kumar Das, coordinator of the COVID-19 management committee of the hospital. “Before the government approved for the import of the injection, the patients used to manage the medicine on their own. But as the injection is available now, we consensually medicate the patients who are in critical condition.”
Some progressive effect of the medicine can be seen in the patients, but the ultimate effectiveness of Remdesvir in patients infected with the novel coronavirus cannot be ascertained as of now, added Dr. Das. “The patients are medicated with Remdesivir from five to 10 days. Since the medicine arrived only few days ago, the patients have not completed the full course of the anti-viral injection yet,” he said.
Dr. Das added that the final outcome of the effectiveness of Remdesivir could be ascertained only after the patients complete the dosage. He also said that the plasma therapy was also being applied on the virus patients along with the medication of Remdesivir.
Meanwhile, Dr. Rabindra Pandey, a public health specialist, said that since the Remdesivir anti-viral was under the third-phase of clinical trial, the medicine was being given to the COVID-19 patients only under their approval.
According to the international protocols, the medicines which are under trial are given to the patients only with their consent, added Dr. Pandey. “Also, if the patients suffer from side effects of the medication, the company manufacturing the medicine shall compensate the patients,” he added. “Although Remdesivir is not the definitive medicine which would cure the novel coronavirus infection, the medicine is working quite effectively here in Nepal,” added Dr. Pandey. “However, it is not as effective as the plasma therapy.”
Recent News

Do not make expressions casting dout on election: EC
14 Apr, 2022
CM Bhatta says may New Year 2079 BS inspire positive thinking
14 Apr, 2022
Three new cases, 44 recoveries in 24 hours
14 Apr, 2022
689 climbers of 84 teams so far acquire permits for climbing various peaks this spring season
14 Apr, 2022
How the rising cost of living crisis is impacting Nepal
14 Apr, 2022
US military confirms an interstellar meteor collided with Earth
14 Apr, 2022
Valneva Covid vaccine approved for use in UK
14 Apr, 2022
Chair Prachanda highlights need of unity among Maoist, Communist forces
14 Apr, 2022
Ranbir Kapoor and Alia Bhatt: Bollywood toasts star couple on wedding
14 Apr, 2022
President Bhandari confers decorations (Photo Feature)
14 Apr, 2022











